When I was a graduate student, my mentor
John Wikswo assigned to me the job of measuring the
magnetic field of a nerve
axon. This experiment required me to dissect the ventral nerve cord out of a
crayfish, thread it through a
wire-wound ferrite-core toroid, immerse the nerve and toroid in
saline, stimulate one end of the nerve, and record the magnetic field produced by the propagating action currents. One day as I was lowering the instrument into the saline bath, a bubble got stuck in the gap between the nerve and the inner surface of the toroid. “Drat” I thought as I searched for a needle to remove it. But before I could poke it out I wondered “how will the bubble affect the magnetic signal?”
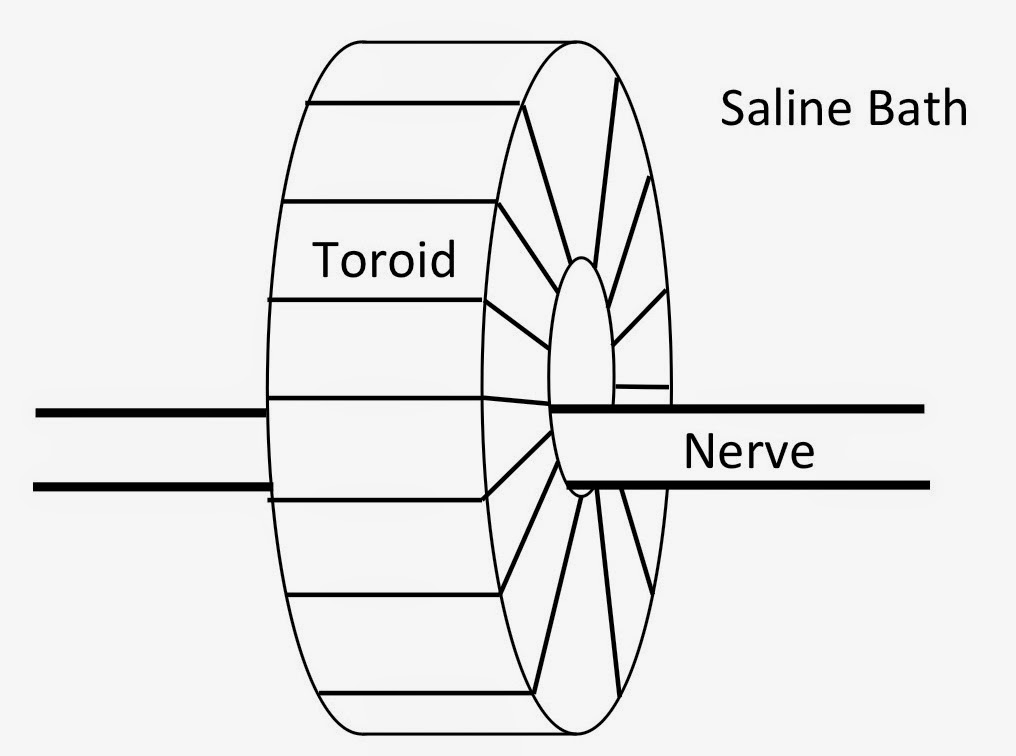 |
A wire-wound, ferrite-core toroid,
used to measure the magnetic field of a nerve. |
To answer this question, we need to review some
magnetism.
Ampere’s law states that the line integral of the magnetic field around a closed path is proportional to the net current passing through a surface bounded by that path. For my experiment, that meant the magnetic signal depended on the net current passing through the toroid. The net current is the sum of the current inside the nerve axon and that fraction of the current in the saline bath that threads the toroid—the return current. In general, these currents flow in opposite directions and partially cancel. One of the difficulties I faced when interpreting my data was determining how much of the signal was from intracellular current and how much was from return current.
I struggled with this question for months. I calculated the return current with a mathematical model involving
Fourier transforms and
Bessel functions, but the calculation was based on many assumptions and required values for several parameters. Could I trust it? I wanted a simpler way to find the return current.
Then along came the bubble, plugging the toroid like
Pooh stuck in
Rabbit’s front door. The bubble blocked the return current, so the magnetic signal arose from only the intracellular current. I recorded the magnetic signal with the bubble, and then—as gently as possible—I removed the bubble and recorded the signal again. This was not easy, because surface tension makes a small bubble in water sticky, so it stuck to the toroid as if glued in place. But I eventually got rid of it without stabbing the nerve and ending the experiment.
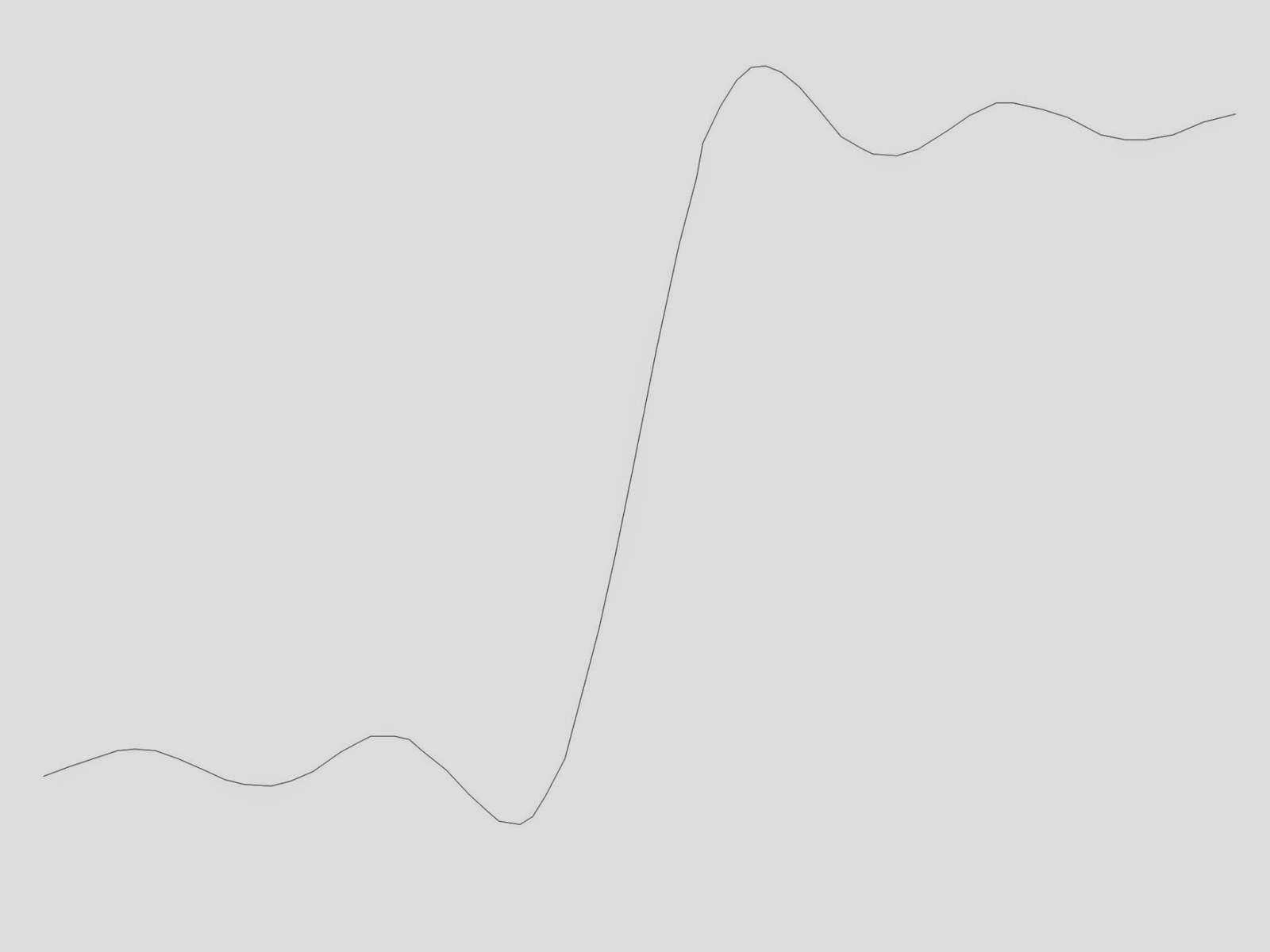
To my delight, the magnetic field with the bubble was much larger than when it was absent. The problem of estimating the return current was solved; it’s the difference of the signal with and without the bubble. I reported this result in one of my first publications (Roth, B. J.,
J. K. Woosley and J. P. Wikswo, Jr., 1985, “An Experimental and Theoretical Analysis of the Magnetic Field of a Single Axon,” In:
Biomagnetism: Applications and Theory, Weinberg, Stroink and Katila, Eds., Pergamon Press, New York, pp. 78–82.).
When taking data from a crayfish nerve, the toroid and axon were lifted
out of the bath for a short time. […] When again placed in the bath an air
bubble was trapped in the center of the toroid, filling the space between
the axon and the toroid inner surface. […] Taking advantage of this
fortunate occurrence, data were taken with and without the bubble
present. […] The magnetic field with the bubble present […] is narrower
and larger than the field with the toroid filled with saline.
 |
The magnetic field of a nerve axon
with and without a bubble trapped
between the nerve and toroid. |
On the day of the bubble experiment I was lucky. I didn’t plan the experiment. I wasn’t wise enough or thoughtful enough to realize in advance that a bubble was the ideal way to eliminate the return current. But when I looked through the
dissecting microscope and saw the bubble stuck there, I was bright enough to appreciate my opportunity. “Chance favors the prepared mind.”
I have a habit of turning all my stories into homework problems. You will find the bubble story in the 4th edition of
Intermediate Physics for Medicine and Biology, Problem 39 of Chapter 8. Focus on part (b).
Problem 39 A coil on a magnetic toroid as in Problem 38 is being used to measure the magnetic field of a nerve axon.
(a) If the axon is suspended in air, with only a thin layer of extracellular fluid clinging to its surface, use Ampere’s law to determine the magnetic field, B, recorded by the toroid.
(b) If the axon is immersed in a large conductor such as a saline bath, B is proportional to the sum of the intracellular current plus that fraction of the extracellular current that passes through the toroid (see Problem 13). Suppose that during an experiment an air bubble is trapped between the axon and the inner radius of the toroid? How is the magnetic signal affected by the bubble? See Roth et al. (1985).